 |
| You're not going to like it, but not all of the protein foods in this photo deserve the attribute "SuppVersity Suggested" |
I usually try to keep my promises,... even if this means hours of research and ending up with the conclusion that "more research is necessary"... and no, you don't have to worry, there's more than that in the following ~4500 words + 45 references (not including those I didn't copy from the reviews I cite): plenty of information about the effects of cooking, storage, temperature changes, physical processing, and - you're lucky - a preliminary list of 8 tips / rules (and
short explanations) that will help you minimize the amount of oxidized protein in your diet, without having to cut back on the intake of your beloved protein ;-)
For those of you who are now wondering PROTOX is / are and why they are (literally) a matter of life or death, I suggest you go back to my previous article about the (ill) health effects of oxidized dietary (!) proteins from Thursday, May 19, 2016 (
read the article | as always, for free!).
This is not an anti-high-protein article. It is one arguing in favor of "treating your protein right"

Are You Protein Wheysting?

5x More Than the FDA Allows!

More Protein ≠ More Satiety

Protein Oxidation = Health Threat

Protein Timing DOES Matter!

More Protein = More Liver Fat?
In view of the fact that this article got longer than the average "101" article, I'd suggest that anyone who is in a hurry
fast forward to the list of eight simple rules I announced in the title of this article. You can bookmark the rest for later, print it to read it in bed or simply rely on my interpretation of the implications of what we know about the various ways processing and storage can damage proteins :
- Cooking, frying, baking, i.e. heat treatment(s), in general - While you don't have to eat your meat, fish and dairy raw, those of you who "fry their proteins to death" and eat their steak well-done are at a higher risk of exposing themselves to exuberant amounts of oxidized proteins than the rare steak conconnoisseurs.
While the number of studies testing for protein oxidation in meats during cooking is higher than those on any other protein source, their number is, compared to the one of studies investigating the oxidation of fats and cholesterol in meat products, still low and their significance is crippled by the use of either inappropriate markers of protein oxidation or different markers that make it virtually impossible to compare the results of one study to another.
An example of the initially mentioned problem, i.e. the use of inappropriate markers of protein oxidation, would be the 1997 study on the effects of different cooking methods on some lipid and protein components of hamburgers by Rodriguez-Estrada, M. T., et al. (1997). Their use of free form amino acids as marker of protein oxidation suffers from the presence of the latter in raw meat and their drip loss during cooking.
 |
| Figure 1: In contrast to the amount of free amino acids, the formation of Schiff bases provides reliable information about the oxidation of protein during e.g. cooking (data from Gatellier. 2010). |
A much better way to estimate the degree of protein oxidation in meat and other high protein foods is the formation / presence of Schiff bases. The latter are precursors to maillard products of which studies indicate that they have divergent effects on human health: with some of them being implicated in the development of diabetes mellitus, cardiovascular complications, and Alzheimer's disease and others ostensibly helping with gut health, inflammation, and chemoprevention (the benefits appear to be mediated by hormesis or the effect of these molecules on the make-up of your microbiome | Smith. 1994; Somoza. 2005; Tuohy. 2006; Tessier. 2012). Measuring these byproducts of reactions between proteins and aldehydic products, as it was done by Gatellier, et al. (2010 | see data in Figure 1), provides significantly more reliable information about the degree of protein oxidation in foods than the measurement of the amount of free amino acids which would suggest that raw meat was more oxidized than cooked meat (cf. Rodriguez-Estrada. 1997), because the drip loss of amino acids is falsely interpreted as a reduction in protein oxidation compared to the raw product. That this is not the case and heating meat and other high protein foods will increase, not decrease the amount of oxidized proteins can be seen in the data from Gatellier, et al. in Figure 1.
Furthermore, Gatellier's and other studies indicate that the formation of protein oxides which starts at relatively low temperatures of only >65°C does not begin to really take off before temperatures of 123-207°C - temperatures as you would see them if you fry your foods and can be reduced (albeit not significantly | 1300% increase vs 3500% increase | cf. Figure 2) if the cattle has been fed anti-oxidant rich diets.
 |
| Figure 2: Effect of diet and cooking parameters on protein oxidative modifications (Gatellier. 2010). |
This is not surprising, as it has long been established that increases in lipoxidation products go hand in hand with increased oxidative damage to proteins (Requena. 1996). Any diet that would modify the concentration of certain lipids and lipid protecting antioxidants (like vitamin E) will thus change both, the oxidation of fats and proteins in high protein foods. The reverse applies to pro-oxidant diets (e.g. with rancid oils), like the one Zhang et al. (2010) used in broiler chickens whose breast meat would then show increased levels of oxidized proteins.
You must not forget, however, that the data in Figure 1 also shows that even the quality L+R meat is not fully protected from oxidation when it is heated at high(er) temperatures for more than 60 seconds - and that's something that is going to happen even for the most bloody (non-raw) steak. "t "and "T", i.e. time and temperature, are thus not the only, but the most significant determinants of protein oxidative modifications (cf. Figure 3).
An increased drip loss suggest increased protein oxidation: It's not just annoying if your meat weights only half of what it weighed before cooking, it is also an albeit not 100% reliable indicator or, I should say, correlate of increased protein oxidation. That's at least what a 2012 study by Traore et al. indicates: in said study, the researchers analyzed the effects of heat treatments on meat from the M. longissimus thoracis from Galia and Redone pigs. What they found was a striking and statistically significant correlation between the presence and level of oxidized proteins and the drip loss during cooking / frying. This observation does not only suggesting a possibly reduced ability of oxidized proteins to retain water, but also the possibility of using the drip loss, which is a marker of meat quality, anyways, as an easily accessible indicator / estimate for protein oxidation.
 |
| Figure 3: Time matters, but heat, too - Markers of protein oxidation in meat exposed to 100°C (grey) and 270°C for the time in minutes indicated on the x-axes (Santé-Lhoutellier. 2008). |
In fact, previous research confirms and underli-nes the importance of time and tempera-ture and indicates that even at temperatures of only 100°C, the level of oxidation products in meat proteins will double within just 5 minutes.
When the time of exposure is short, the corresponding +200% increase in carbonyls (a marker of PROTOX | see Figure 3) that occurs at low temperatures is allegedly relatively small. If you are into slow-cooking your meats at "low" temperatures for several minutes, any low temp. advantage would be lost, though.- Traditional processing methods to increase the storage time like dry-curing or fermentation - In contrast to the effects of heat treatment(s), the ones of other common processing methods are comparably under-researched. What we do know, however, is that dry-curing meats (and probably dry-aging, too - although a study on that has still to be done, to answer your Facebook question honestly, Matthew) can lead to sign. hydrolytic degradation of proteins (Toldrá, 1998). Furthermore, recent studies indicate that meat proteins will also undergo different, often more intense (compared to proteolysis) oxidative reactions during dry curing (Estévez. 2011).
 |
| Figure 4: Carbonyl content (nmoles/mg protein) in longissimus dorsi (unprocessed) and dry-cured loins from different pigs on diefferent diets; % indicates relative increase in protein oxidation (Ventanas. 2006). |
Studies carried out by Ventanas et al. (2006 & 2007) prove the presence of significant amounts of protein carbonyls in dry-cured loins and hams - a process of which the researchers study shows that it was, once again, linked to the lipid oxidative reactions that occur during curing.
 |
| Steaming uses temps >60°C where proteins start to oxidize and steaming takes its time. It is thus not per se safe. |
Steaming is not the solution: Unfortunately, detailed comparisons of the effects of steaming, microwave cooking, smoking, grilling, frying, and other cooking methods are not available - temperature level and duration of heat treatment have been the major focus in the literature and the results indicate that even "low" temperatures, such as the 100°C of steam will induce significant damage to meat, poultry or fish proteins during the 5-10 minutes they have to be exposed to the thermic treatment; that's because even at temperatures only slightly >60 °C can cause the oxidative cleavage of the porphyrin ring, which in turn will result in an increased release of heme iron, which has been shown to drive both, lipid and protein oxidation (Miller. 1994).
- The hams, which are subjected to longer and more severe drying conditions, were found to have considerably larger amounts of protein carbonyls than the loins (≈ 9 nmol/mg protein vs. ≈ 1.3 nmol/mg protein). The manufacture of dry-cured meats involves a lengthy process (up to 36 months for Iberian dry-cured hams) and several operations such as salting, post-salting, drying and cellar (Ventanas. 2007). Little is known about the impact of each step on the onset and intensity of the oxidative reactions affecting to meat proteins. What studies have shown, however, is that ..
"[s]alting, which is a common operation for the manufacture of numerous meat products, may have an impact on protein carbonylation. The addition of sodium chloride has an impact on the ionic strength of the environment which in turn, affects the degree of assembly of MP (Wick, 1999) their exposure to pro-oxidants and hence, their susceptibility to carbonylation (Montero, Giménez, Pérez-Mateos, & Gómez-Guillén, 2005). In addition, several authors have proposed that NaCl could enhance the activity of Fe3+ or that Cl− derived from NaCl would improve the solubility of such ion, hence, stimulating their pro-oxidant effects (Kanner et al., 1991 and Osinchak et al., 1992)" (Estévez. 2011).
Accordingly, you can assume that the protein oxidation increases with the amount of salt that's used when meat or, as the study by Osinchak et al. shows, fish is cured - an interesting observation that should remind you of the link between high salt intakes and heart disease, of which said link suggests that it could at least partly be mediated by the effect of salt on protein oxidation in processed meats and other high protein foods.
- Storage, cooling, freezing, high pressure and irradiation to reduce bacterial contamination, and packaging - You already know that cooked protein products have increased carbonyl levels compared to raw samples (1–3 nmol/mg protein in raw vs. 5 nmol/mg protein in cooked products), but the heat exposure is not the only threat to the proteins' integrity: cutting, mincing, and all the other processing steps protein foods undergo will likewise make significant contributions to the formation of protein carbonyls and other processes of protein oxidation.
 |
| Figure 5: Evolution of protein oxidation in liver pâtés from Iberian and white pigs (note the difference that exists even before storage) under refrigerated storage (Estévez & Cava. 2004). |
In contrast to the former processing steps, the effects of subsequent refrigerated storage, has also been extensively studied - with disconcerting results, namely significant increases in the concentration and oxygenation of non-heme iron and thus the extent of lipid and protein oxidation (Estévez & Cava, 2004) which can render certain meat product, like liver pâtés that are refrigerated for several months quasi non-suitable for human consumption (see Figure 5) and will still significantly increase the protein oxidation of other less processed foods w/ lower amounts of iron such as chicken legs and breasts (see Figure 6)
 |
| Figure 6: Changes in carbonyl content in chicken (a) leg meat and (b) breast meat as
affected by freezing temperature and 6 months of storage at different freezing temperatures (Soyer. 2010). |
Now, freezing, which is particularly damaging if it is done at rather low temperatures at home (cf. Figure 6), is not the only storage method that increases the protein carbonyl content of meat and other high protein foods. In fact, storage per se will trigger natural biochemical processes that will inevitably favor protein oxidation. In that, decreases in pH and increases in the concentration of H+ ions are only one of various drivers of oxidative stress that can increase the susceptibility to protein carbonylation / oxidation - processes that will be further accelerated when meat is irradiated (common practice in the US meat industry) to kill bacteria (compare the size of the orange with the blue bars in Figure 7).
 |
| Figure 7: Effect of Storage Time (12 Days) on Nonirradiated and Irradiated Raw Chicken Breast Meats Stored at 5 °C on Carbonyl Content as Lipid (in µmol Acetophenone/10 g Meat | Rababah. 2004) |
It is thus not surprising that Martinaud, et al. (1997) observed a significant increase of the carbonyl content in meat from beef longissimus lumborum and diaphragma pedialis muscles: from 3.1 to 5.1 nmol/mg protein and from 4.8 to 6.9 nmol/mg protein, respectively, during only 10 days of chill storage. Subsequent studies confirmed the occurrence of protein carbonylation during aging/chill storage of beef, pork, poultry, turkey, lamb, rhea, ostrich meat and eggs, in which Liu et al. (2009) found a 60% increase in egg white and a 41% increase in egg yolk protein oxidation.
Speaking of eggs, ... the change of the textural and structural quality of egg yolk and white upon heating is not the only change egg proteins will undergo while being heated at temperatures above 70 °C at atmospheric pressure or being exposed to high pressure of >500−600 MPa which will trigger the oxidative formation of disulfide bonds among the egg proteins (Van der Plancken. 2005 & 2006). That doesn't change my conclusion that the presence of
oxysterols (oxidized cholesterol) is probably of greater health relevance than the lack of heat stability of egg proteins.
- Due to the large variability of the data reported by different authors for the amount of protein carbonyls in similar meat samples analyzed, it is unfortunately not possible to infer general patterns (Estévez. 2011) - that's also because the "extent of protein carbonylation is highly dependent on the origin of the meat, type of muscle, species and the storage [and feeding] conditions" (Estévez. 2011).
Another thing that appears to be proven is the fact that beef is significantly susceptible to protein carbonylation than pork - a fact scientists ascribe to the noticeably larger amounts of iron and myoglobin in cattle muscles (Lund. 2007a,b). Accordingly, it can hardly surprise you that a similarly lower propensity for protein oxidation has been found by Mercier et al. (1998) when they compared beef and turkey meat.
 |
| Figure 8: Determinants of the susceptibility of meat(s) and poultry to protein oxidation. |
If the differences are in fact, as the scientists argue, due to the different amounts of iron and myoglobin in various muscle meats, it is not just logical that (a) beef is more susceptible to protein oxidation than pork, but also that (b) pork more prone to protein oxidation than poultry and that (c) gylcolytic, low fat, low myoglobin (strength) type muscle meat is less susceptible to carbonylation than oxidative, high fat, high myoglobin (endurance type) muscle meat (Filgueras et al., 2010) - how oxidized the protein in your meats is will thus depend on both, species and the cuts you choose (+ other previously discussed factors).
 |
| Figure 9: Levels of AAS (a) and GGS (b) in dry-cured ham as analysed by LC–MS. Mean values correspond to area units from the peak integration of EIC for [M+H]+267 (AAS–ABA) and for [M+H]+253 (GGS–ABA) and corrected considering the total protein content of each batch. Different letters on the bars denote statistical differences (p < 0.05) amongst means. IF (intact format), CSF (conventional-sliced format) and ASF (alternative-sliced format - which also influence markers of protein oxid. | Fuentes. 2010). |
How significant the effects of other emerging processing technologies such as the application of hydrostatic pressure, which is used as an alternative to irradiation and pasteurization to kill bacteria, eventually is, has not yet been fully elucidates. While studies by Cava et al. (2009) and Fuentes et al. (2010) indicate that the application of high pressure can causes a significant increase on the amount of specific protein carbonyls, α-aminoadipic and γ-glutamic semialdehydes (AAS and GGS), "as a likely result of physico-chemical changes induced by hydrostatic pressure including tissue disruption and increase of free catalytic iron" (Estévez. 2011), the evidence is far from being conclusive and the pressure in your pressure cooker (~100 MPa or 15 psi) is 6 times lower than the 600 MPa used by Cava et al. (2009) and applied for a 100x shorter time periods than it took to produce significant protein oxidation in Cava et al. (2009) at 300 MPa. So the pressure in a pressure cooker is probably less of an issue than the time your meats and other protein sources will spend in it at high temperatures.
The same goes for the commonly used modified atmosphere packaging (MAP), which has been shown to contribute to increased myosin cross-links, and increased protein carbonyls especially if the "modified atmosphere" is high in oxygen (70% to 80% | Lund 2007a,b) - a condition that has been shown to reduce the formation of thiol groups, i.e. fat oxidation, but has the obvious downside of increasing protein oxidation. Alternative packaging methods would be available, 100% nitrogen (results are mixed, e.g. Zanardi. 2002; Leygonie. 2011) or vacuum-packaging (effectively reduced protein oxidation during storage compared to MAP | Lagerstedt. 2011), for example, but just like the use of additives and ingredients with proven antioxidant capacity they are rarely used and more research is necessary to determine which of them offers the best protection to both proteins and fat.
- None-meat, -poultry or -fish proteins react similarly - While there's lots of research on protein oxidation in meats, studies on other food sources are scarce, but show similar trends with respect to processing-induced protein oxidation.
In cheeses, for example, the lowest amounts of protein oxidation are found in "unripe" raw milk cheeses or cheese that was produced from milk that was semi-cooked or pasteurized at low (vs. high as in UHT milk) temperatures (Fedele. 2001).
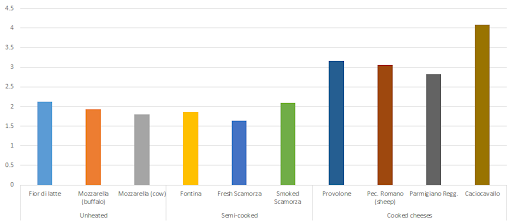 |
| Figure 10: Protein-bound carbonyls (PC) in selected cheeses from differently processed milk, i.e. unheated, semi-cooked and cooked milk as a raw ingredient (Fedele. 2001). |
With the processing temperature, the amount of protein-bound carbonyls (PC), the previously discussed marker of protein oxidation, increases, significantly. In Figure 10 illustrates that pretty well, after all, the cheeses that were made from cooked milk all have sign. higher PC levels than those from raw or semi-cooked milk. Much in contrast to meats, the storage or, in the case of cheeses, ripening processes does not trigger a continuous increase in protein oxidation (data not shown): For Grana Padano, a cheese that's produced from cooked milk, for example, the rapid (+30%) increase in protein carbonyls in the first 1-3 months of ripening does not continue and the level of PCs remains relatively stable for the rest of the several months period spanned by the study by Fedele et al.
 |
| Figure 11: Kinetics of protein carbonyl generation by UV or fluorescent photooxidation. Protein carbonyls were determined in milk
exposed to UV or fluorescent (FL) light, as a function of time of exposure to radiation.
■ = whole milk (WM) exposed to FL light; ● = skim milk (SM) exposed
to FL light; □ = WM exposed to UV light; and ◯ = SM exposed
to UV light (Scheidegger. 2010) |
Next to high temperature exposure (and yes, there will be some protein oxidation during pasteurization, but the process is fast enough to make the effects negligible | Table 1), UV radiation is a se-cond threat to dairy proteins.
As the data in Figure 11 goes to show you, it takes only a few hours under a 15W UV lamp and/or a similarly "weak" fluorescent lamp to trigger rapid increases in protein oxidation in "milk", or rather what Scheidegger, et al. call milk, i.e. a liquid that was produced from water with commercially processed spray-dried whole milk powder, of which you can already expect that it has sign. higher baseline PC levels than regular milk.
To deliver milk in regular, opaque tetra packs instead of the hipster glass bottles is thus a very good idea! Much in contrast to the use of regular or hypoallergic milk powders in processed and baby foods, the consumption of sweet condensed milk or its unsweetened alternative, evaporated milk.
 |
| Table 1: Lysine damage in commercial milk samples (Mauron. 1990). |
Just like roller dried milk powder which is used in chocolates, and spray-dried lactose-hydrolized milk powder of which I have to admit that I don't know in which processed junkfoods it is used, the former food ingredients harbor significant amounts of blocked lysine, a previously discussed marker of protein oxidation that occurs relatively early during the oxidation process.
Corresponding data for vegetable proteins is unfortunately not available. Even though vegetable proteins usually don't come with high amounts of easily oxidizable lysine (casein has a 2.0 ratio of lysine to arginine, soy's ratio is 0.9 | Kritchevsky. 1979), iron and myoglobin, soy, pea, hemp, bean and other vegan / vegetarian protein sources are not immune to processing, i.e. heat-, light-, and pressure-induced protein oxidation or the ongoing deterioration of their protein structure during storage. Whether these effects are strong enough to turn an originally antioxidant or neutral food protein into a pro-oxidant dietary ingredient, will yet need further clarification in realistic scenarios. Initial evidence that this could be the case comes from a 2015 study by Chen et al. who administered heat-oxidized soy protein to broiler chickens and observed that it would impair the chickens growth performance - probably as a result of negative effects on the digestive function.
 |
| Don't be a fool: There's no evidence that protein powders are "adulterated" with exorbitant amounts of protein oxidation products; and, even more importantly, the currently available evidence in favor of the beneficial health effects of whey and co. clearly refute the practical health significance of the most likely existing amounts of protein oxidation products in the average protein powder. |
Where's the info about whey, casein and other protein powders? In view of the fact that I can only report data that exists, it is very difficult for me to answer one of the most frequently asked questions I got after the publication of my original article on protein oxidation, namely:
"What about my whey protein?"
What I have found out, though, is that whey proteins, like any other protein food, undergo heat-induced protein damage. For whey protein concentrates, Rector et al. report a rapid degradation of protein complexes when the protein is stored above the critical 60-65°C margin (Rector et al. used 80°C). Storage at 25°C for 1 year, on the other hand, resulted in polymerization of "only" 18% of the monomeric beta-lactoglobolins, and did not involve the formation of intermolecular cross-links, a characteristic feature of protein oxidation (Morr. 1993).
Whether the amount of protein oxidation products that
certainly exist in casein, whey, soy, pea, and other protein powders is health-relevant, is therefore highly questionable. Furthermore, the well- and widely-established antioxidant effects of whey, which include, among other things, a reduction of protein oxidation in vivo (e.g. Haraguchi. 2011), would suggest that that the average whey protein doesn't just compensate, but
over-compensate any potentially pro-oxidative effects of existing protein oxides by the anti-oxidant effects of its various antioxidant proteins and peptides (Tong. 2000; Peña‐Ramos. 2004; Peng. 2009).
In this context it may also be worth mentioning that a study by Fenaille et al. (2006), which investigated the protein-carbonyl (PC) levels in infant protein powder formulas, and is thus the study that comes closest to an analysis of PC levels in commercially available protein powders, found the amount of oxidized proteins to be not much different from those in unprocessed, cold-stored meat products. If certain quality standards during the production are met, it is thus unlikely that any potential worries you may have that protein powders could be much unhealthier than meat or other protein sources are warranted.
Wow, that was a lot of information on what you want to do or not do to reduce the oxidation of proteins. Since skipping on all protein containing foods and/or resorting only to raw meats, fish and milk is probably not an option. I have tried to use the previous information to phrase a few suggestions on what to do and not to do in order to limit your PROTOX exposure:
 |
| Figure 11: Influence of freeze–thaw cycles on TBARS (fat) & carnonyl (protein oxid.) in pork (Xia. 2009) |
buy, cook and eat fresh - it doesn't take heat to damage proteins, storage - even at very low temperatures - will also induce significant increases in protein oxidation; accordingly it makes sense to buy meat, poultry and fish fresh and to avoid storing and refrigerating them (esp. in view of the fact that storage / refrigeration in regular refrigerators will be more damaging than refrigeration of meats in much more efficient industrial refrigerators | Figure 11)- avoid irradiated and or high pressure treated, industrially processed meats and prefer vacuum packaged meats over meats in modified atmosphere packaging w/ extra high oxygen levels (nitrogen is no problem) - the less processed and messed up the meat you buy, the less significant any further increases in protein oxidation you may induce at home will be; you should also ask your butcher if he already froze and thawed the allegedly "fresh" meat he offers - you may not believe it, but much of the "fresh" meat has actually been refrigerated and thawed at least once - especially, the more exotic cuts; also, vacuum packaging your meats at home makes as much sense as any other way of repackaging it to keep them fresh - none of them is going to be worse than simply putting the meat into the fridge in nothing but a simple plastic bag; pressure cooking is, as discussed previously probably not an issue (due to the comparatively low pressure), but the time your meats and other protein sources spend in the pressure cooker at relatively high temperatures could be
- use culinary herbs and spices - even though there is no data for each and every culinary herb and spice, the data that exists shows sign. reductions in protein, fatty acid oxidation and the formation of advanced glycation end-products (AGEs) for cloves, ground
cinnamon, ground Jamaican allspice, apple pie spice, oregano, ground
pumpkin pie spice, marjoram, sage, thyme, gourmet Italian
tarragon, mint, rosemary, Italian
poultry seasoning, turmeric, curry powder, chili powder, basil, nutmet, ginger, parsley, black pepper, and rosemary (Dearlove. 2008; Haak. 2008);
 |
| Figure 12: Protein hydrazones (expressed as nmol hydrazones/mg protein) gain during refrigeration of cooked burger patties with added fruit extracts and quercetin (Ganhão. 2010). |
and using high polyphenol oils like virgin olive oil for cooking and in marinades may further reduce the protein oxidation; speaking of which, marinades and or fillings and the addition made from antioxidant-rich fruits or plant products (e.g. onions, garlic and other high quercitin foods that mimic the addition of pure quercitin in Ganhao (2010) | Fig. 12) have also been shown to help minimize protein oxidation not just during cooking, but also during storage
- appreciate quality, non-cured meat - a high drip loss (meaning the weight of your meat is significantly reduce when you cook it) is a common marker of low meat quality and a correlate of high protein oxidation (Traore. 2012); the same goes for the loss of water that occurred way before you bought the meat during curing; and, yes, this means if there's a significant source of oxidized proteins in your diet, your beef jerky, bacon and other cured meats are the prime suspects - not only, but especially if they're also high in salt
- choose low(er) fat meats, poultry, fish, dairy and vegetable protein over iron- and myoglobin-laden red meats - it's not just the lower iron and myoglobin content of poultry and fish that makes them more resilient to protein oxidation, it is also their low(er) fat content, which reduces potential cross-reactions of oxidized fats with proteins; this does not mean that you cannot eat fatty cuts of red meat, at all, but there's more than just epidemiological evidence that the consumption of high amounts of processed and/or improperly stored or packaged red meat is problematic; not just, but also because of high levels of oxidized proteins
 |
| Don't do everything the print on these packs tells you: While you certainly don't want to eat these silica packets, you also don't want to throw them away before you emptied your protein tub - they will help to keep your protein (and other supplements) dry! |
store your protein powders cool, dry, and by any means not in the sun - there's no evidence that protein powders could be a major source of oxidized proteins in your diet - unless you do your best to make them go rancid by (a) rehydrating and storing them as an RTD, which will significantly increase the amount of volatile compounds even if the product is cooled (Park. 2016 / storing the protein with or at least not removing the silica gel packets (see photo on the right) may also help), (b) storing your protein powders in transparent containers in the sun, or (c) putting your protein tubs right next to a radiator or any other heat source- prefer raw or pasteurized over ultra-high temperature processed (UHT) milk / dairy products - in contrast to what the scaremongering on the internet would suggest the short application of relatively low temperatures (72°C) during the pasteurization process does not induce significant damage to the protein-structure of dairy products; that's in contrast to UHT, which is done with at least 53°C higher temperatures and is notorious for its effects on the protein structure - an effect you will even taste and smell (Clare. 2005)
- go easy on the heat and keep the duration of any heat exposure short - "well-done" or "tar-black" are words you shouldn't be forced to use to describe your dietary protein sources; you also don't want to simmer your meats and other high protein foods at 100°C (or even >65°C) for hours or fool yourself to believe that steaming your fish at 100°C for 10 minutes was so much better (in terms of protein oxidation) than frying it for 2 minutes
 |
| Figure 13: A comparison of the initial and progressing oxidation of myoglobin (data expressed as % of total myoglobin) in intact and minced slices of beef gluteus medius samples (3 mm thick) that were observed by Ledward & MacFarlane observed in their 1971 study clearly suggests that (a) the process of mincing induced protein oxidation and (b) makes the meat more susceptible to progressing protein oxidation during storage. |
Any Facebook-questions left? Yes, the thing about
gelatin. Well, I have to admit that I neither know why one would even remotely consider consuming gelatin in amounts that would make it worth worrying about its oxidized protein content, nor whether gelatine even contains oxidized protein. In view of the fact that EAAs like lysin appear to promote protein oxidation just like iron and myoglobin, and considering the fact that gelatin contains neither of them in sign. amounts, I do yet have my doubts that gelatin is a sign. source of PROTOX in your diet, Gillian.
And Kirill, reliable data on the protein-carbonyl content of
ground vs. non-ground beef is not available. The increased surface (=more oxygen exposure = iron / hemoglobin oxidation) and the heat that is produced when you mince it, would suggest ground beef will have higher higher amounts of oxidized proteins. Evidence that this hypothesis is accurate comes from a 1971 study by Ledward & MacFarlane who observed higher levels of oxidized myoglobin in minced vs. intact beef (see
Figure 13).
Now, before I leave you totally confused about your diet, let me add this: Neither this, nor the
previous article I wrote about the health effects of protein oxidation should be misunderstood as anti-protein propaganda. Protein oxidation is, after all, only one out of a myriad factors that will determine the health effects of your diet. If you know about it and take the previously outlined measures to keep your intake of oxidized proteins in check, there's no good reason to assume that the occurrence of oxidized proteins in animal, vegetable or other protein sources would be reason enough to sign. limit their intake, let alone avoid them altogether |
Comment!
References:
- Chen, Nannan, Mouming Zhao, and Weizheng Sun. "Effect of protein oxidation on the in vitro digestibility of soy protein isolate." Food chemistry 141.3 (2013): 3224-3229.
- Chen, X., et al. "Effects of heat-oxidized soy protein isolate on growth performance and digestive function of broiler chickens at early age." Asian-Australasian journal of animal sciences 28.4 (2015): 544.
- Clare, D. A., et al. "Comparison of sensory, microbiological, and biochemical parameters of microwave versus indirect UHT fluid skim milk during storage." Journal of dairy science 88.12 (2005): 4172-4182.
- Dearlove, Rebecca P., et al. "Inhibition of protein glycation by extracts of culinary herbs and spices." Journal of medicinal food 11.2 (2008): 275-281.
- Estévez, Mario. "Protein carbonyls in meat systems: A review." Meat Science 89.3 (2011): 259-279.
- Estévez, Mario, and Ramón Cava. "Lipid and protein oxidation, release of iron from heme molecule and colour deterioration during refrigerated storage of liver pâté." Meat Science 68.4 (2004): 551-558.
- Fedele, E., and P. Bergamo. "Protein and lipid oxidative stresses during cheese manufacture." Journal of food science 66.7 (2001): 932-935.
- Fenaille, Françis, et al. "Modifications of milk constituents during processing: A preliminary benchmarking study." International Dairy Journal 16.7 (2006): 728-739.
- Filgueras, R. S., et al. "Colour, lipid and protein stability of Rhea americana meat during air-and vacuum-packaged storage: Influence of muscle on oxidative processes." Meat science 86.3 (2010): 665-673.
- Ganhão, Rui, David Morcuende, and Mario Estévez. "Protein oxidation in emulsified cooked burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage." Meat science 85.3 (2010): 402-409.
- Gatellier, Ph, et al. "Effect of cooking on protein oxidation in n-3 polyunsaturated fatty acids enriched beef. Implication on nutritional quality." Meat science 85.4 (2010): 645-650.
- Haak, Lindsey, et al. "Effect of dietary rosemary and α-tocopheryl acetate on the oxidative stability of raw and cooked pork following oxidized linseed oil administration." Meat Science 78.3 (2008): 239-247.
- Han, J., and K. S. Rhee. "Antioxidant properties of selected Oriental non-culinary/nutraceutical herb extracts as evaluated in raw and cooked meat." Meat science 70.1 (2005): 25-33.
- Kanner, Joseph, et al. "Lipid peroxidation of muscle food: the role of the cytosolic fraction." Journal of agricultural and food chemistry 39.2 (1991): 242-246.
- Kritchevsky, David. "Vegetable protein and atherosclerosis." Journal of the American Oil Chemists’ Society 56.3 (1979): 135-140.
- Lagerstedt, Åsa, Kerstin Lundström, and Gunilla Lindahl. "Influence of vacuum or high-oxygen modified atmosphere packaging on quality of beef M. longissimus dorsi steaks after different ageing times." Meat Science 87.2 (2011): 101-106.
- Ledward, D. A., and J. J. Macfarlane. "Some observations on myoglobin and lipid oxidation in frozen beef." Journal of Food Science 36.7 (1971): 987-989.
- Leygonie, C., T. J. Britz, and L. C. Hoffman. "Protein and lipid oxidative stability of fresh ostrich M. Iliofibularis packaged under different modified atmospheric packaging conditions." Food Chemistry 127.4 (2011): 1659-1667.
- Liu, X. D., et al. "Effect of irradiation on foaming properties of egg white proteins." Poultry science 88.11 (2009): 2435-2441.
- Lund, Marianne N., et al. "High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage." Meat Science 77.3 (2007a): 295-303.
- Lund, Marianne N., Marchen S. Hviid, and Leif H. Skibsted. "The combined effect of antioxidants and modified atmosphere packaging on protein and lipid oxidation in beef patties during chill storage." Meat Science 76.2 (2007b): 226-233.
- Martinaud, Agnès, et al. "Comparison of oxidative processes on myofibrillar proteins from beef during maturation and by different model oxidation systems." Journal of Agricultural and Food Chemistry 45.7 (1997): 2481-2487.
- Mauron, Jean. "Influence of processing on protein quality." Journal of nutritional science and vitaminology 36.4-SupplementI (1990): S57-S69.
- Mercier, Y., et al. "Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in turkey meat during storage." Meat Science 48.3 (1998): 301-318.
- Miller, D. K., et al. "Dietary iron in swine rations affects nonheme iron and TBARS in pork skeletal muscles." Journal of food science 59.4 (1994): 747-750.
- Montero, P., et al. "Oxidation stability of muscle with quercetin and rosemary during thermal and high-pressure gelation." Food Chemistry 93.1 (2005): 17-23.
- Morr, C. V., and E. Y. W. Ha. "Whey protein concentrates and isolates: processing and functional properties." Critical Reviews in Food Science & Nutrition 33.6 (1993): 431-476.
- Nieto, Gema, et al. "Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary, or garlic." Meat science 95.2 (2013): 177-184.
- Osinchak, Joanne E., et al. "Effect of NaCl on catalysis of lipid oxidation by the soluble fraction of fish muscle." Free Radical Biology and Medicine 12.1 (1992): 35-41.
- Peña‐Ramos, E. Aida, Youling L. Xiong, and Guillermo E. Arteaga. "Fractionation and characterisation for antioxidant activity of hydrolysed whey protein." Journal of the Science of Food and Agriculture 84.14 (2004): 1908-1918.
- Peng, Xinyan, Youling L. Xiong, and Baohua Kong. "Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance." Food Chemistry 113.1 (2009): 196-201.
- Rababah, Taha, et al. "Effect of electron beam irradiation and storage at 5 C on thiobarbituric acid reactive substances and carbonyl contents in chicken breast meat infused with antioxidants and selected plant extracts." Journal of agricultural and food chemistry 52.26 (2004): 8236-8241.
- Rector, D., N. Matsudomi, and J. E. Kinsella. "Changes in Gelling Behavior of Whey Protein Isolate and β‐Lactoglobulin During Storage: Possible Mechanism (s)." Journal of food science 56.3 (1991): 782-788.
- Rodriguez-Estrada, M. T., et al. "Effect of different cooking methods on some lipid and protein components of hamburgers." Meat science 45.3 (1997): 365-375.
- Santé-Lhoutellier, Veronique, et al. "Effect of meat cooking on physicochemical state and in vitro digestibility of myofibrillar proteins." Journal of Agricultural and Food Chemistry 56.4 (2008): 1488-1494.
- Scheidegger, D., et al. "Protein oxidative changes in whole and skim milk after ultraviolet or fluorescent light exposure." Journal of dairy science 93.11 (2010): 5101-5109.
- Smith, Mark A., et al. "Advanced Maillard reaction end products are associated with Alzheimer disease pathology." Proceedings of the National Academy of Sciences 91.12 (1994): 5710-5714.
- Somoza, Veronika. "Five years of research on health risks and benefits of Maillard reaction products: an update." Molecular nutrition & food research 49.7 (2005): 663-672.
- Soyer, Ayla, et al. "Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat." Food chemistry 120.4 (2010): 1025-1030.
- Tessier, Frédéric J., and Ines Birlouez-Aragon. "Health effects of dietary Maillard reaction products: the results of ICARE and other studies." Amino acids 42.4 (2012): 1119-1131.
- Tong, Lawrence M., et al. "Mechanisms of the antioxidant activity of a high molecular weight fraction of whey." Journal of Agricultural and Food Chemistry 48.5 (2000): 1473-1478.
- Traore, S., et al. "Effect of heat treatment on protein oxidation in pig meat." Meat science 91.1 (2012): 14-21.
- Tuohy, Kieran M., et al. "Metabolism of Maillard reaction products by the human gut microbiota–implications for health." Molecular nutrition & food research 50.9 (2006): 847-857.
- Van der Plancken, Iesel, Ann Van Loey, and Marc EG Hendrickx. "Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment." Journal of agricultural and food chemistry 53.14 (2005): 5726-5733.
- Van der Plancken, Iesel, Ann Van Loey, and Marc E. Hendrickx. "Effect of heat-treatment on the physico-chemical properties of egg white proteins: A kinetic study." Journal of Food Engineering 75.3 (2006): 316-326.
- Ventanas, Sonia, et al. "Protein and lipid oxidation in Longissimus dorsi and dry cured loin from Iberian pigs as affected by crossbreeding and diet." Meat Science 72.4 (2006): 647-655.
- Ventanas, S., et al. "Extensive feeding versus oleic acid and tocopherol enriched mixed diets for the production of Iberian dry-cured hams: Effect on chemical composition, oxidative status and sensory traits." Meat Science 77.2 (2007): 246-256.
- Vuorela, Satu, et al. "Effect of plant phenolics on protein and lipid oxidation in cooked pork meat patties." Journal of Agricultural and Food Chemistry 53.22 (2005): 8492-8497.
- Wu, Wei, et al. "Oxidative modification of soy protein by peroxyl radicals." Food Chemistry 116.1 (2009): 295-301.
- Xia, Xiufang, et al. "Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles." Meat Science 83.2 (2009): 239-245.
- Zanardi, E., et al. "Lipid and colour stability of Milano-type sausages: effect of packing conditions." Meat Science 61.1 (2002): 7-14.




























